AEvice Health (Singapore)
For those who follows up with news around the international start-up scene, you would find this name really familiar. AEvice Health is frequently featured in tech news, like here, here and here. Late last year, they were also part of the Channel NewsAsia TV programme, Season 4 of Start-UP and were recently awarded in the Lee Kuan Yew Global Business Plan Competition.
With such widespread publicity, AEvice Health is definitely the start-up to look out for.
Intelligent Asthma Monitoring Device
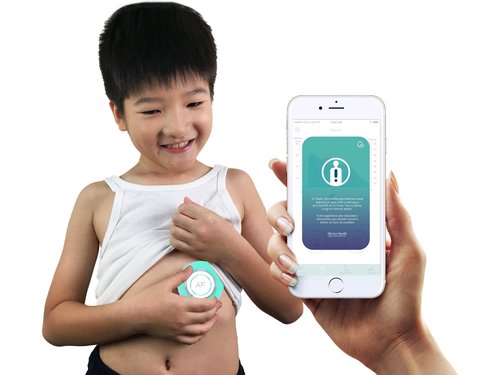
AEvice Health develops wearable devices that helps doctors today to better diagnose asthma, and for patients to better self-manage their condition. They are currently developing an intelligent asthma monitoring device (BioAsthma), which will be launched on June 2018.
BioAsthma is a wearable device, which continuously monitors your child’s asthma condition. It uses state of the art technology to detect vital signs such as wheezing, breathing pattern and coughs for better asthma management.
We are fortunate enough to speak with CEO & Founder, Mr Adrian Ang, about their journey and the challenges they face from the angle of quality and regulatory angle.
Largest obstacles in ISO 13485
With BioAsthma targeted to be commercialised by December next year, the management team is putting their focus on ensuring a high quality and regulatory adherence throughout the entire process of design and development.
“The most challenging part of ISO 13485 is not knowing what you don’t. As such, we might be vulnerable for non-conformance during audits
— Adrian Ang, when being asked what is their biggest challenge by far.
Fortunately, AEvice Health is blessed with great friends and mentors from the same sector, who shared about the importance of quality and adhering to regulatory requirements at an earlier stage. “Despite having prior experience in setting up and operating quality management system in my previous career, ISO 13485 is still relatively fresh to me and my team. As a result, we actively look out for guidance from our peers in this industry”, Adrian added.
“It is rare to see companies being aware of the daunting requirements of ISO 13485. AEvice Health was ready for it and had segmented a part of their project fund for such purpose, which is going to help accelerate their product into the market when it is launched. There were many before them that weren’t as lucky, as they were unaware of the regulatory landscape, causing them to run into roadblocks during the ISO 13485 journey – often causing painful delays”, CEO of Stendard, Jason Lim shared.
Existence of Stendard First™
“With Stendard First™, we got the customised documents that are applicable to us within about 2 hours of use”, Adrian estimated. The next move was to then read and fully understand the boundaries and requirements of ISO 13485.
As these documents were fully customised to AEvice Health, precious time and money were saved to focus on the right things, learning to implement the generated documents into the company’s processes.
Stendard First™ is essentially designed to focus on the upstream portion of the entire ISO 13485 journey – the document creation process. Watch introductory video of Stendard First™. (WATCH VIDEO)
When asked about additional support that they require from Stendard, Adrian suggested “It would be perfect if Stendard can assign some advisors’ help to review our document and confirm that we have completely got the documents right, so that we can jump right into implementation after that.”
Document Review and Implementation
With the Stendard First™ already supporting more than 20 companies within a month’s launch, it’s easy to wonder about what’s upcoming for Stendard.
To give all our blog followers a sneak peek, we are currently in late stage discussion to work with our top tier partners to provide Document Review supports.
At an affordable price, we will support early-stage companies to go through:
- Gap Analysis – To understand the current stage of company and progress of commercial products
- Document Creation – Wholly supported by Stendard First™ and guided by an attached advisor
- Document Review – To complete the process by ensuring the document creation is fully customised and applicable to the company
- Training and Implementation (Optional) – To put all theoretical processes into practical application through several intensive sessions of training.
To find out more, fill out the enquiry form stating your requirements.
Moving Forward
Every year, among the 38,000 ISO 13485:2016 certifications issued worldwide, there are many just like AEvice Health Pte Ltd, who felt restricted and limited in resources. One word of advice, try out Stendard First™.




